Abstract
Introduction: Diffuse large B-cell lymphoma not otherwise specified (DLBCL NOS) is divided into germinal center B-cell (GCB) and activated B-cell-like (ABC) molecular subtypes, which differ both biologically and clinically, ABC DLBCL often presenting with less favorable outcome. The clinical course of DLBCL is also impacted by the tumor microenvironment (TME). However, differences in the composition of the TME between the GCB and ABC DLBCLs have not been uncovered.
Materials and Methods: We analyzed gene expression from 81 primary DLBCL samples using the Nanostring nCounter Human PanCancer Immunoprofiling Panel, and characterized B cells (CD20), T cells (CD3, CD4, CD8, FOXP3, TBET, Granzyme B (GrB)), macrophages (CD68, CD163), NK cells (CD56, GrB), and immune checkpoint molecules expressed on these cells (PD-1, PD-L1, TIM3) from 178 tumors using multiplex immunohistochemistry (mIHC). In addition, we measured HLA-ABC, HLA-DR, and B2M expression immunohistochemically. Finally, we performed cell-cell interaction analyses (Brück et al. Mod Pathol. 2021). The patients were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)-like immunochemotherapy, and the findings correlated with patient demographics and survival.
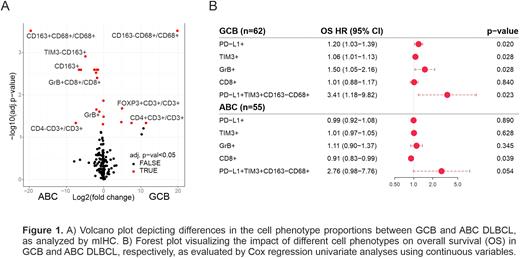
Results: As expected, differential gene expression analysis between the molecular subtypes revealed that ABC DLBCLs expressed significantly higher levels of TNFRSF13B, IRF4 and AICDA. In addition, genes related to macrophage signaling, such as CD163, MARCO and CD47 were upregulated. In contrast, GCB DLBCLs expressed higher levels of genes associated with T cell receptor signaling, such as ICOSLG, HLA-DOB, IL4 and CD40LG. Based on mIHC (Figure 1A), CD163+ tumor associated macrophages (TAMs) were enriched in the ABC DLBCL TME (adj. p < 0.001), whereas in the GCB DLBCL TME, TAMs were more often CD163- (adj. p < 0.001). In the GCB DLBCL TME, a higher proportion of all tumor-infiltrating T cells were T helper cells (CD4+; adj. p = 0.023), and regulatory T cells (FOXP3+; adj. p = 0.026), while GrB+ cytotoxic cells were more abundant in the ABC subtype (adj. p = 0.006). Consistently, ABC DLBCLs were more frequently HLA-ABC and B2M positive compared to GCB DLBCLs (p = 0.005 and p = 0.002, respectively).
Interestingly, in ABC DLBCL, CD163+ TAMs interacted more often with T cells, other TAMs, and B cells than in GCB DLBCL (adj. p < 0.001 for all). Instead, in GCB DLBCL, T cells shared more interactions with other T cells and macrophages (adj. p < 0.001 for all). However, while T cells had generally more interactions in GCB DLBCL, cytotoxic T cells had more cell interactions in ABC DLBCL (adj. p < 0.001).
The prognostic impact on tumor-infiltrating immune cells on survival varied between the two molecular subtypes (Figure 1B). High proportion of immune checkpoint-expressing TAMs associated with unfavorable survival in both molecular subtypes. However, in the patients with GCB DLBCL, high proportion of PD-L1+, TIM3+ and GrB+ cells predicted unfavorable survival, whereas in the patients with ABC DLBCL, high proportion of CD8+ cytotoxic T cells associated with favorable outcome.
Conclusions: The TME of GCB and ABC-like DLBCL are distinct by composition, cell-cell interaction patterns and in terms of prognostic biomarkers.
Disclosures
Leppa:Gilead Sciences: Consultancy, Honoraria; BMS: Consultancy, Research Funding; Bayer: Research Funding; Orion Pharma: Consultancy; Pfizer: Consultancy; Beigene: Consultancy; Genmab: Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Nordic Nanovector: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.